Understanding Allene: Unraveling The Chemistry Behind "Allene And Mollie Hari Where Are They Now"
Many people, it seems, are curious about "Allene and Mollie Hari where are they now," and that's a very interesting search query. You might be wondering about individuals, perhaps public figures, or even a duo known for something special. However, it's really important to clarify something right from the start: based on the information we have, "Allene" is actually a fascinating chemical compound, not a person. And as for "Mollie Hari," well, that name doesn't appear in the scientific context of Allene at all. This article will focus on the chemical "Allene" and its current standing in the world of science, helping to shed light on what "Allene" is all about and why it's so important in chemistry today.
It's quite common for names to have multiple meanings, isn't it? In this particular case, the "Allene" you're likely encountering in scientific discussions refers to a unique type of organic molecule. This molecule holds a special place in chemistry because of its distinctive structure and the way it behaves in various reactions. So, if you were hoping to find a celebrity or a historical figure named Allene, you might be a bit surprised, but what you'll learn about this chemical is actually pretty cool, too.
Our aim here is to give you a clear picture of what Allene is from a scientific viewpoint, explaining its structure, how it's used, and what recent discoveries have been made about it. We'll explore its unique atomic arrangements and how chemists use it to build more complex molecules, giving you a good sense of its importance in the lab. This way, you'll have a better grasp of where "Allene" is in the scientific landscape right now, which is really what "where are they now" means in this context.
- St Cloud Fl Mayor Race
- Parade Of Paws Rescue
- Carrie Keagan Erome
- Washington Street Skate Park Photos
- Ai Power 2025 Event Hong Kong Venue
Table of Contents
- Understanding Allene: A Chemical Profile
- Allene in Action: Powerful Chemical Reactions
- Allene Oxide Cyclase (AOC): A Biological Connection
- Frequently Asked Questions About Allene
- The Future of Allene Chemistry
Understanding Allene: A Chemical Profile
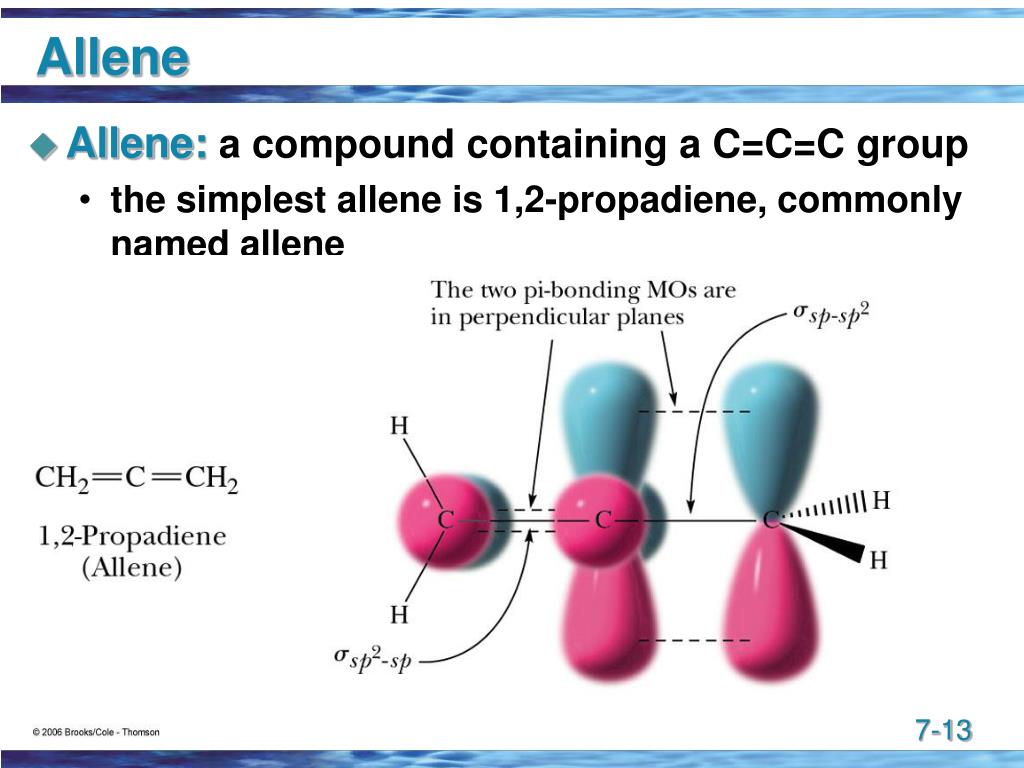
So, what exactly is "Allene" in the world of chemistry? Basically, it's a type of organic compound, a hydrocarbon to be precise, that has a very distinct arrangement of carbon atoms. It's known for having two double bonds that are right next to each other, which chemists call "cumulative double bonds." This specific setup gives Allene some rather special properties that make it a valuable building block in many chemical reactions. It's truly a fascinating molecule to study, you know.
Allene's Unique Structure
When we look closely at Allene's structure, we find something quite interesting about its carbon atoms. Allene (Taylor, 1967) has two sp2 hybridized carbons located at its ends. These sp2 carbons are typically found in molecules with double bonds, allowing for a flat, trigonal arrangement around them. But the real twist is the central carbon atom, which is sp hybridized. This sp hybridization means the central carbon forms two linear bonds, which is a bit different from what you might usually see in simple organic molecules.
The presence of these different hybridization states, that is, sp2 at the ends and sp in the middle, creates a very particular geometry for the molecule. What's more, the two cumulative pi bonds, which are formed by the overlap of p-orbitals, exist in planes perpendicular to each other. This perpendicular arrangement is key to Allene's reactivity and is quite unusual, making it a very special case among organic compounds. It's almost like having two separate flat sheets of paper twisted at a right angle to each other, if you can imagine that.
- 58 Bridge Ave Bay Head Nj
- Creole Soul Photos
- Paige Maddux Husband
- The Ultimate Prom And Bridal
- Roadhouse Momo And Grill Photos
Key Features of Allene: A Summary
To give you a quick overview of this intriguing molecule, here's a summary of its main characteristics based on what we know. This information helps us understand why Allene behaves the way it does in various chemical settings, and why it's a subject of ongoing research. It's quite a fundamental piece of chemistry, actually.
| Feature | Description |
|---|---|
| Chemical Type | Hydrocarbon with cumulative double bonds |
| End Carbons | sp2 hybridized |
| Central Carbon | sp hybridized |
| Pi Bonds | Two cumulative pi bonds existing in perpendicular planes |
| Discovery/Description | Described by Taylor in 1967 (referring to structural analysis) |
| Reactivity | Highly reactive due to unique structure, used in various synthetic reactions |
Allene in Action: Powerful Chemical Reactions
Allene's unique structure doesn't just make it interesting to look at; it also makes it incredibly useful in organic synthesis. Chemists use Allene as a starting material to create more complex and valuable molecules through a variety of sophisticated reactions. These reactions often take advantage of Allene's unusual bonding to build rings or attach new groups in very precise ways. It's pretty amazing what you can do with it.
Asymmetric Catalytic (4 + 3) Cycloaddition
One of the most powerful and convenient ways Allene is used is in asymmetric catalytic (4 + 3) cycloaddition reactions. This type of reaction is very important because it allows chemists to build complex ring structures with a specific three-dimensional orientation, which is known as asymmetry. Controlling asymmetry is a huge deal in drug development and materials science, as it can drastically change how a molecule interacts with biological systems or how a material performs. The fact that Allene can be used in such a precise way makes it a truly valuable tool, so it's almost always a topic of discussion in advanced synthesis labs.
Radical Functionalization of Allenes
Another area where Allene chemistry has seen significant progress is in its functionalization via radical processes. This involves using highly reactive species called radicals to add new chemical groups to the Allene molecule. Different radical partners, including carbon radicals, can be used, allowing for a wide range of new compounds to be formed. This approach offers a powerful way to introduce diverse functionalities into molecules, which is vital for creating new materials or medicines. Researchers are constantly finding new ways to make these reactions work more efficiently, and this review, as a matter of fact, focuses on recent advances in this particular area.
Transition-Metal Catalyzed Hydroarylation
The intermolecular transition-metal catalyzed hydroarylation of allenes has also been widely explored, particularly since the early 1980s. This reaction involves adding an aryl group (a type of aromatic ring structure) and a hydrogen atom across the Allene double bonds, using a transition metal as a catalyst. What's special about this reaction is that it allows for the selective formation of new carbon-carbon bonds, which is a fundamental step in building larger organic molecules. The ability to do this selectively means chemists can guide the reaction to produce exactly the desired product, which is incredibly important for efficiency and reducing waste in chemical synthesis. It's a rather elegant way to combine different molecular pieces.
Allene Oxide Cyclase (AOC): A Biological Connection
While Allene itself is a synthetic building block, its derivatives, particularly allene oxides, play a crucial role in biology. This brings us to an enzyme called Allene Oxide Cyclase (AOC). Enzymes are biological catalysts that speed up specific chemical reactions in living organisms. The discovery and study of AOC reveal a fascinating natural pathway involving Allene-related structures. It's quite remarkable how chemistry connects to life processes, you know.
AOC Discovery and Occurrence
Allene oxide cyclase (AOC) was first described from Zea mays, which is corn, in 1995. Following this initial discovery, the corresponding genes for AOC were successfully cloned from tomato in 1996 and from Arabidopsis in 1998. Southern analysis and genetic mapping techniques further revealed that in tomato, a single gene for Allene oxide cyclase exists, located on chromosome 2. The inspection of the N-terminus of the enzyme also provided important clues about its structure and function. This widespread presence in plants suggests a vital role for AOC in plant biology, perhaps in defense mechanisms or growth regulation, though the text doesn't explicitly state the exact biological function, it implies a significant one.
The Role of Allene Oxides
It's interesting to note that allene oxides were known in chemistry well before they were discovered to occur in biology. This means chemists had been studying these molecules in the lab for some time before their natural existence and biological importance became clear. The chemical oxidation of an allene group, which involves adding oxygen across its two double bonds in series, creates these allene oxides. Their presence in plants, catalyzed by AOC, highlights a remarkable example of nature using complex chemical structures for its own purposes. It really shows how interconnected synthetic chemistry and natural processes can be.
Frequently Asked Questions About Allene
Given the nature of the initial search query, it's clear there might be some confusion about what "Allene" actually is. Here are some common questions that people might have about this chemical compound, helping to clear up any misunderstandings and provide more insight into its scientific relevance. We hope these answers are helpful, too.
Is Allene a person or a chemical?
Allene is a chemical compound. Specifically, it's an organic molecule characterized by two double bonds that are right next to each other, known as cumulative double bonds. The name might sound like a person's name, but in the scientific context we're discussing, it refers to this particular chemical structure.
What makes Allene's structure unique?
Allene's structure is unique because its central carbon atom is sp hybridized, while the two carbons at its ends are sp2 hybridized. This arrangement causes the two pi bonds in the molecule to be in planes that are perpendicular to each other. This distinct geometry gives Allene special reactivity and makes it valuable in various chemical reactions, like those used to build complex molecules. It's a bit like a twisted ladder, if you can picture that.
Why is Allene important in chemistry?
Allene is important in chemistry because its unique structure makes it a versatile building block for creating more complex organic molecules. It's used in powerful synthetic methods like asymmetric catalytic cycloadditions, radical functionalization, and transition-metal catalyzed hydroarylation. These reactions allow chemists to precisely construct new compounds with specific properties, which is crucial for developing new drugs, materials, and other useful substances. Its biological derivatives, like allene oxides, also play roles in plant biochemistry, showing its broader significance, too.
The Future of Allene Chemistry
The study of Allene and its reactions remains a very active and exciting field in organic chemistry. Researchers are continuously finding new ways to harness its unique structure for innovative synthetic transformations. The ability to precisely control the addition of new groups to Allene, whether through catalytic methods or radical processes, opens up countless possibilities for creating molecules with specific functions. This ongoing exploration is pushing the boundaries of what's possible in chemical synthesis, which is pretty exciting.
Furthermore, the biological relevance of Allene derivatives, such as allene oxides and the enzyme Allene Oxide Cyclase, suggests there's still much to learn about their roles in natural systems. Understanding these biological pathways could lead to new insights into plant development, defense mechanisms, or even the creation of natural products with therapeutic potential. The connection between synthetic chemistry and natural processes is a fertile ground for discovery, and Allene stands right at that intersection, as a matter of fact.
So, while "Allene and Mollie Hari where are they now" might have initially led you down a path of personal inquiry, we hope this journey into the world of chemical Allene has been informative and engaging. Its story is one of fascinating structure, powerful reactions, and a surprising connection to the living world. To learn more about organic chemistry and its many amazing compounds, you can always explore further on our site. And if you're curious about specific reaction types, you might find more information on catalysis in organic synthesis.
The field is always moving forward, with new discoveries being made all the time. For instance, recent reports continue to refine our understanding of how Allene's unique geometry influences its reactivity in very subtle ways. This constant evolution means that the "where are they now" for Allene is always a story of progress and new applications, keeping it at the forefront of chemical innovation. For more detailed scientific information on allenes, you might want to check out resources like the IUPAC Gold Book, which provides definitions for chemical terms. You can find it here.
- Marina City Club Photos
- Cole Young Metalwood
- Tassi Araujo Pelada
- Autumn Nelson Big Ass
- Ts Jenny Wonders

What is the molecular geometry of Allene and why? | Homework.Study.com
.png)
C3h4 3d Structure
PPT - Alkynes PowerPoint Presentation, free download - ID:861329