Unlocking Chemistry: Your Guide To The Molarity Of Solutions Color By Numbers Answer Key
Ever felt like chemistry problems were a puzzle with missing pieces? Especially when it comes to figuring out the strength of a solution, which we call molarity? Well, you are certainly not alone in that feeling, and it's almost a universal experience for students starting out. It can seem a bit tricky at first, trying to wrap your head around moles, liters, and all those numbers. But what if learning this important concept could actually be a bit more like a fun game?
That's where "color by numbers" activities come into the picture for chemistry. They offer a really neat way to practice these calculations in a very visual and engaging manner. It makes the whole process less intimidating and, quite frankly, a lot more enjoyable, too.
Today, we're going to explore the core ideas behind molarity, look at how these engaging color-by-numbers sheets work, and, perhaps most importantly, help you truly understand how to use a **molarity of solutions color by numbers answer key** not just to check your work, but to truly master the subject. We'll go through some actual examples from my text, so you can see how it all fits together, like your favorite puzzle pieces.
- Bronte London Restaurant
- Mommas Grocery Wine Photos
- Dan Taylor Surfboards
- Matt Walker Mx
- Nate Pontious Age
Table of Contents
- What Exactly is Molarity?
- Why "Color by Numbers" for Molarity?
- Tackling Molarity Problems: A Step-by-Step Guide
- Decoding Your "Molarity Color by Numbers Answer Key"
- Beyond the Basics: Related Concepts
- Frequently Asked Questions About Molarity
- Getting Started with Molarity Mastery
What Exactly is Molarity?
So, what is molarity anyway? It's really just a way chemists talk about how concentrated a solution is. Think of it like this: if you're making lemonade, you might want to know how much lemon juice is in each glass. In chemistry, molarity tells us how many "moles" of a dissolved substance, called the solute, are present in every liter of the entire solution. It's a very precise measurement, and that's why it's so important.
My text tells us directly: "Molarity is defined as moles of solutes in one litre of solution." It's pretty straightforward when you look at it that way. This definition is really the bedrock for all molarity calculations, so it's good to keep it in mind.
Breaking Down the Molarity Formula
The formula for molarity is quite simple, actually, and you'll see it everywhere. It's typically written as M = n/V. Here, 'M' stands for molarity, 'n' is the number of moles of the solute, and 'V' is the volume of the solution, which absolutely must be in liters. You know, it's just one of those things you have to remember.
For instance, my text states: "Molarity = 1 mol 1 l = 1 mol/l = 1 M." This means if you have one mole of a substance dissolved in one liter of solution, its molarity is 1 M. It's a fundamental concept, and you'll find it applies to pretty much all solutions, too.
Moles and Volume: The Core Ingredients
Understanding moles is really key here. A mole is just a specific quantity of a substance, a bit like how a "dozen" means twelve. My text emphasizes, "There is only one definition of a mole," which is true. It's a fixed amount, specifically Avogadro's number of particles, and it helps us count very tiny atoms and molecules in a practical way. You know, it's kind of amazing how we can do that.
Volume, on the other hand, is usually measured in liters for molarity calculations. If you're given milliliters, you'll need to convert them to liters by dividing by 1000. For example, 20 ml becomes 0.020 liters. This conversion is a very common step in these types of problems, so it's good to practice it.
Why "Color by Numbers" for Molarity?
You might be wondering, "Why use a 'color by numbers' activity for something as serious as chemistry?" Well, it's actually a really clever teaching tool. Instead of just solving problems on a plain worksheet, you get to see your progress visually. Each correct answer reveals a part of a picture, which can be very motivating, and that's pretty cool.
It turns a potentially dry subject into something more interactive and less like just another set of equations. For many people, especially those who learn best visually, this approach can make a huge difference in how well they grasp the material. It's not just about getting the right answer, but about the process itself, too.
Making Chemistry Fun and Clear
These activities break down complex problems into smaller, manageable steps. Each calculation you do leads to a specific number, which then corresponds to a color. This immediate feedback helps reinforce your learning. If your picture isn't turning out right, you know you need to recheck your calculations, and that's a pretty clear signal.
It helps build confidence, especially when you start seeing the image come together. It shows you that you are, in fact, getting the answers right, and that feels good. Plus, it's just a bit more fun than traditional worksheets, isn't it? It's kind of like solving a puzzle, but with a scientific twist, you know?
Tackling Molarity Problems: A Step-by-Step Guide
Let's walk through some examples, using the information from my text, to see how molarity calculations work in practice. This will help you understand the types of problems you might encounter in a "color by numbers" activity, and how to approach them systematically. It's really about breaking things down into smaller parts, that's all.
Example 1: Potassium Iodide Calculation
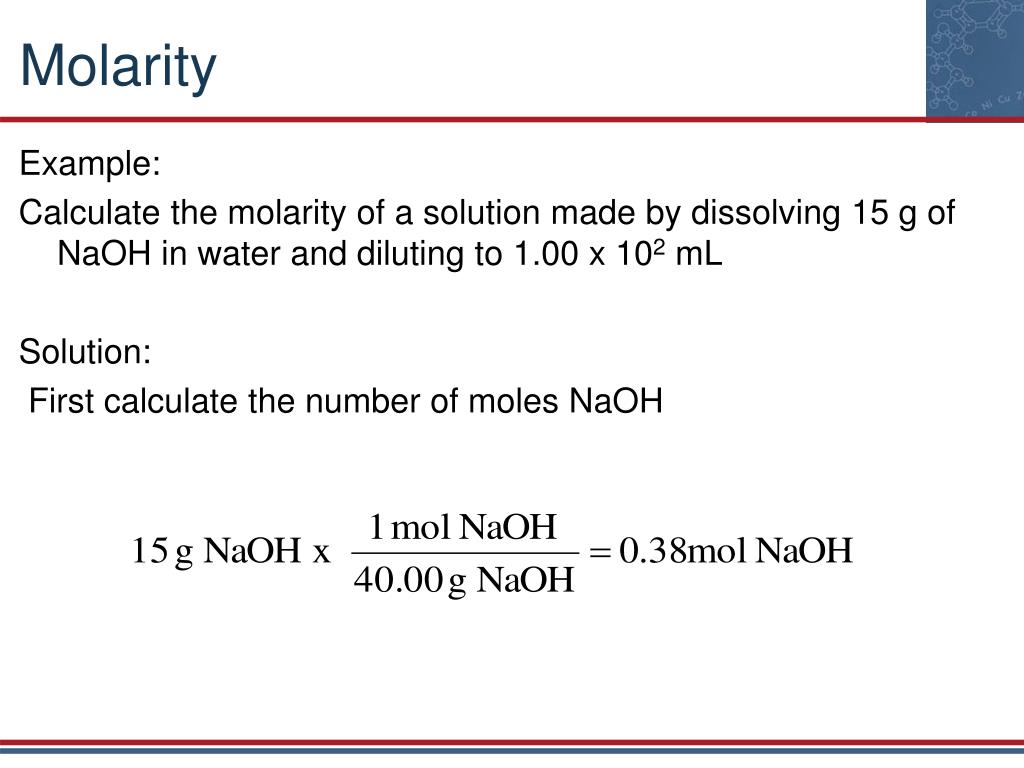
My text asks: "How do i calculate the molarity of 7.24 * 10^2 ml of solution containing 22.4 g of potassium iodide?" This is a classic molarity problem where you're given mass and volume, and you need to find molarity. First, we need to convert the volume from milliliters to liters. 7.24 * 10^2 ml is 724 ml, which is 0.724 liters. That's a very important first step.
Next, we need the number of moles of potassium iodide (KI). To do this, you'd typically need the molar mass of KI. Let's say the molar mass of K is about 39.1 g/mol and I is about 126.9 g/mol. So, KI's molar mass is roughly 39.1 + 126.9 = 166.0 g/mol. Then, moles = mass / molar mass = 22.4 g / 166.0 g/mol = approximately 0.135 moles of KI. Finally, Molarity = moles / volume = 0.135 mol / 0.724 L = approximately 0.186 M. See, it's just a few steps, really.
Example 2: Preparing a Sodium Chloride Solution
Another scenario from my text is: "How do we prepare a 500 ⋅ ml volume of 0.077 ⋅ mol ⋅ l−1 solution with respect to sodium chloride?" Here, you're given the desired volume and molarity, and you need to figure out how much solute (NaCl) you need. This is essentially working the molarity formula backward, which is pretty common.
First, convert the volume: 500 ml is 0.500 liters. Now, use the molarity formula (M = n/V) to find moles (n = M * V). So, n = 0.077 mol/L * 0.500 L = 0.0385 moles of NaCl. To prepare the solution, you'd then convert these moles to grams using the molar mass of NaCl (Na ≈ 23.0 g/mol, Cl ≈ 35.5 g/mol, so NaCl ≈ 58.5 g/mol). Mass = moles * molar mass = 0.0385 mol * 58.5 g/mol = approximately 2.25 grams of NaCl. You see, it's just about rearranging the formula a little bit.
Understanding Molar Mass for Conversions
My text mentions needing to "convert 3 mol cacl2 to mass in grams" and also refers to "the molar mass of iron (ii) nitrite, fe(no2)2." This really highlights the importance of molar mass. It's the mass of one mole of a substance, and you find it by adding up the atomic masses of all the atoms in a chemical formula. It's a very crucial step for moving between moles and grams, which you'll do a lot in chemistry, so you know, it's worth getting good at.
For instance, if you have 3 moles of CaCl2, and you know the molar mass of Ca is about 40.1 g/mol and Cl is about 35.5 g/mol, then CaCl2 would be 40.1 + (2 * 35.5) = 40.1 + 71.0 = 111.1 g/mol. So, 3 moles of CaCl2 would be 3 mol * 111.1 g/mol = 333.3 grams. This calculation is quite direct, and it's something you'll use constantly when working with solutions, actually.
Decoding Your "Molarity Color by Numbers Answer Key"
So, you've done your calculations, you've colored in your picture, and now you want to check your work with the **molarity of solutions color by numbers answer key**. This key is not just for copying answers; it's a powerful learning tool if you use it correctly. It's basically your guide to making sure you're on the right track, and that's pretty helpful.
The answer key will typically show the final colored image or list the correct answers for each problem, corresponding to a specific color. Your goal is to compare your work to this key, not just to see if you got the right color, but to understand why. It's about learning from any mistakes you might have made, which is, you know, how we all get better.
How to Use an Answer Key Wisely
When you look at the answer key, don't just glance at it. Take your time. If a section of your picture doesn't match the key, go back to that specific problem. Rework it step-by-step. Did you convert milliliters to liters correctly? Did you calculate the molar mass accurately? Did you use the right formula? It's about identifying where your thought process might have gone a little off, you know?
It's also a good idea to try to explain your steps out loud to yourself or to a friend. This process of articulation can often reveal where your understanding might be a bit fuzzy. The answer key is a guide, not a shortcut. It's there to help you learn, not just to give you the answers, so it's a very valuable resource.
Common Pitfalls and How to Avoid Them
One very common mistake is forgetting to convert volume to liters. My text specifically mentions that molarity volume is "in litres." This is a big one, and it's easy to overlook when you're rushing. Always double-check your units. Another pitfall is miscalculating molar mass. A small error there can throw off your entire molarity calculation, and that's not what you want.
Also, sometimes people mix up moles of solute with liters of solute. My text reminds us, "You cannot use molarity to find liters of solute," which is a really important distinction. Molarity relates moles of solute to the total volume of the *solution*. Being mindful of these small details can save you a lot of trouble, and it's worth paying attention to them.
Beyond the Basics: Related Concepts
Once you're comfortable with molarity, you'll find it connects to many other important chemistry concepts. For example, my text mentions "osmolarity," which is related to molarity but takes into account how many particles a substance breaks into when dissolved. This is especially important in biological systems, and it's just another way molarity helps us understand solutions, you know?
You'll also see molarity in stoichiometry problems, like the balanced chemical equation mentioned in my text: "agn o3(aq) +kcl(aq) → agcl(s) +kn." Molarity helps you figure out how much of one reactant you need to completely react with another. It's a very fundamental building block for more advanced chemistry topics, so getting a good grip on it now is a pretty smart move.
For instance, my text also touches on redox reactions, like "(c r2o7)2− + f e2+ = c r3+ + f e3+." In these reactions, molarity is crucial for calculating concentrations and reaction yields. Understanding molarity really opens up a lot of doors in chemistry, and it's a concept you'll use again and again. You can learn more about chemistry calculations on our site, and link to this page here for additional practice problems.
Frequently Asked Questions About Molarity
How do you find the answer to molarity color by numbers?
You typically find the answer by performing the molarity calculations for each problem presented on the sheet. Each correct numerical answer will correspond to a specific color, which you then use to fill in a section of the picture. The "answer key" itself is usually a separate sheet showing the completed, correctly colored image or a list of answers and their corresponding colors, so you can check your work against it. It's a bit like a treasure hunt, really, where the treasure is a colorful picture.
What is the formula for molarity?
The main formula for molarity is M = n/V. In this formula, 'M' stands for molarity, which is expressed in moles per liter (mol/L). The 'n' represents the number of moles of the solute, which is the substance being dissolved. And 'V' stands for the total volume of the solution, which absolutely must be in liters. This formula is the cornerstone of molarity calculations, and you'll use it all the time, so it's very helpful to memorize it.
How do you calculate moles from grams?
To calculate moles from grams, you need to use the molar mass of the substance. The molar mass is the mass of one mole of a compound, usually found by adding up the atomic masses of all the atoms in its chemical formula from the periodic table. The formula for this conversion is: Moles = Mass (in grams) / Molar Mass (in g/mol). For example, if you have 22.4 g of potassium iodide (KI), you'd divide that by KI's molar mass (around 166.0 g/mol) to get the number of moles. This step is pretty essential for many chemistry problems, including those involving molarity, so it's a good skill to have.
Getting Started with Molarity Mastery
Learning molarity doesn't have to be a struggle. With tools like "color by numbers" activities and a clear understanding of how to use their answer keys effectively, you can really build your confidence and knowledge. Remember, it's about understanding the "why" behind the answers, not just getting them right. Keep practicing, keep asking questions, and you'll find that chemistry can be quite fascinating, too. You know, it's pretty rewarding when it all clicks.
For more detailed information on chemical calculations and definitions, you might find resources like Purdue University's Chemistry Help very useful. They often provide clear explanations that can complement your learning, and that's a good thing.
Two-Step Equations Color by Numbers | Answer Key for Teachers | Perfect

Molarity Worksheet Answer Key – Pro Worksheet
PPT - Chapter 16 Solutions PowerPoint Presentation, free download - ID